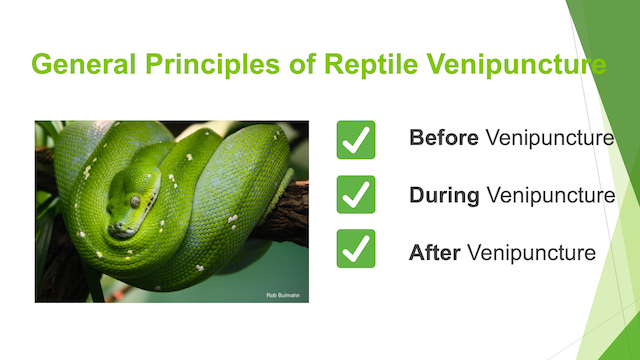
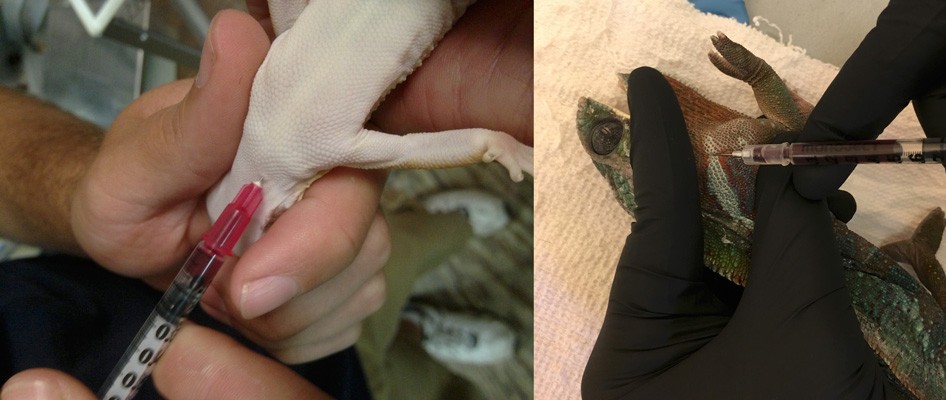
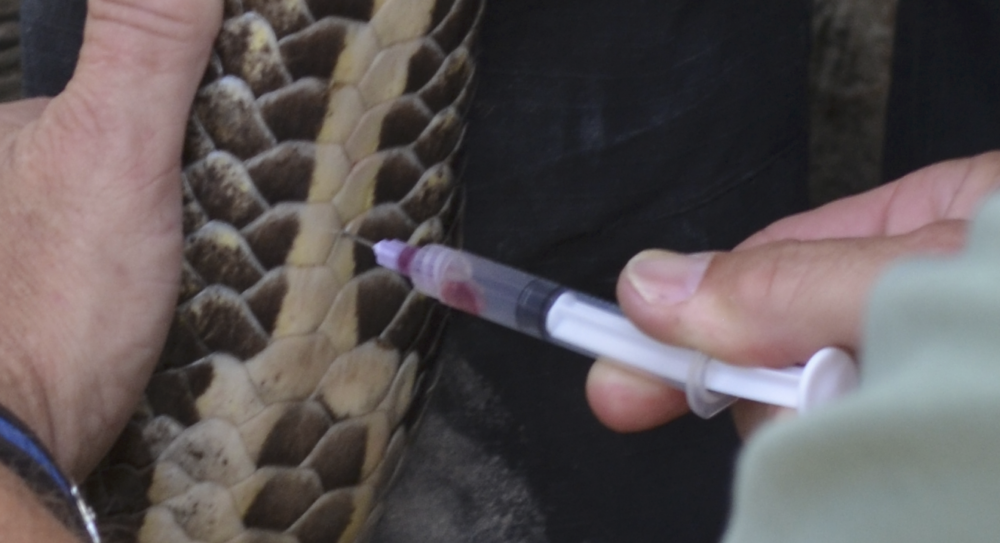
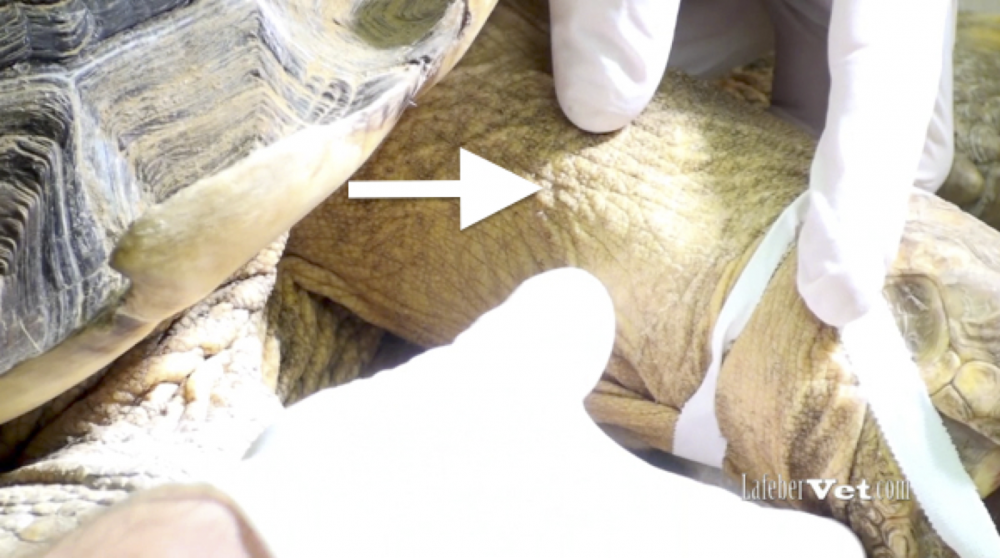
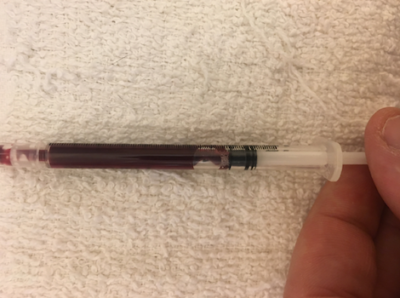
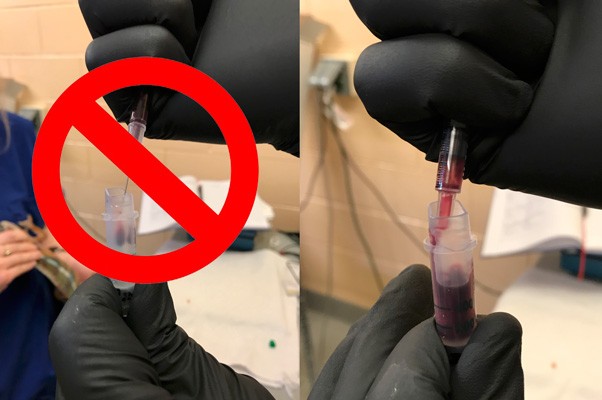
Hematology and biochemistry results serve as an important part of the minimum database for all veterinary patients. Although collection of blood samples can be a clinical challenge in reptiles, the method of patient handling, blood collection and sampling techniques are all critical for proper interpretation of laboratory results. This brief video or slideshow with still images reviews the basic principles of reptile venipuncture that should be considered before, during, and after the procedure . . .
Pour les vétérinaires. Par les vétérinaires.
Le site Lafervet.com est conçu pour une utilisation par les vétérinaires. Il est ouvert aux vétérinaires diplômés, aux techniciens vétérinaires diplômés, aux animaliers et aux étudiants dans ces domaines.
Créer un compte pour accéder à des articles et des ressources du site.
L'inscription est gratuite.
Para Profesionales Veterinarios. Por Profesionales Veterinarios.
El sitio Lafervet.com es para uso de los profesionales veterinarios. Está abierto a los veterinarios licenciados, técnicos veterinarios licenciados, rehabilitadores licenciados y estudiantes en estos campos.
Cree una cuenta para acceder a los artículos y recursos del sitio.
La registro es gratis.
Already a LafeberVet Member?
Please Login
References
1. Berry KH, Demmon A, Bailey T. Protocols for drawing blood from the brachial plexus of desert tortoises. USGS Website. July 2005. Available at https://www.werc.usgs.gov/fileHandler.ashx?File=/…/Protocolbloodnasalrev0705.pdf. Accessed September 27, 2016.
2. Bogan JE, Antonio F, Zachariah TT, Stacy NI. Comparison of the effects of three anticoagulants on hematological analytes in the Eastern indigo snake (Drymarchon couperi). J Herp Med Surg. 2020;30(2):96–100. doi: 10.5818/17-07-118.2.
3. Bonnet X, El Hassani MS, Lecq S, et al. Blood mixtures: impact of puncture site on blood parameters. J Comp Physiol B. 2016;186(6):787-800. doi: 10.1007/s00360-016-0993-1. Epub 2016 May 4. PMID: 27146147.
4. Divers SJ. Diagnostic techniques and sample collection. In: Divers SJ, Stahl SJ (eds). Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed. St. Louis, MO: Elsevier. 2019:1085-1091.
5. Eisenhawer E, Courtney CH, Raskin RE, Jacobson E. Relationship between separation time of plasma from heparinized whole blood on plasma biochemical analytes of loggerhead sea turtles (Caretta caretta). J Zoo Wildl Med. 2008;39(2):208-215. doi: 10.1638/2007-0166R.1. PMID: 18634211.
6. Gottdenker NL, Jacobson ER. Effect of venipuncture sites on hematologic and clinical biochemical values in desert tortoises (Gopherus agassizii). Am J Vet Res. 1995;56(1):19-21. PMID: 7695143.
7. Hanley CS, Hernandez-Divers SJ, Bush S, Latimer KS. Comparison of the effect of dipotassium ethylenediaminetetraacetic acid and lithium heparin on hematologic values in the green iguana (Iguana iguana). J Zoo Wildl Med. 2004;35(3):328-32. doi: 10.1638/03-057. PMID: 15526887.
8. Harr KE, Raskin RE, Heard DJ. Temporal effects of 3 commonly used anticoagulants on hematologic and biochemical variables in blood samples from macaws and Burmese pythons. Vet Clin Pathol. 2005;34(4):383-388. doi: 10.1111/j.1939-165x.2005.tb00065.x. PMID: 16270264.
9. Heatley JJ, Russell KE. Hematolgy. In: Divers SJ, Stahl SJ (eds). Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed. St. Louis, MO: Elsevier; 2019:857-865.
10. Heatley JJ, Russell KE. Clinical chemistry. In: Divers SJ, Stahl SJ (eds). Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed. St. Louis, MO: Elsevier; 2019:899-909.
11. Johnson JG, Nevarez JG, Beaufrere H. Effect of manually preheparinized syringes on packed cell volume and total solids in blood samples collected from American alligators (Alligator mississippiensis). J Exot Pet Med. 2014;23(2):142-146. doi: 10.1053/j.jepm.2014.02.008.
12. Klein K, Gartlan B, Doden G, et al. Comparing the effects of lithium heparin and dipotassium ethylenediaminetetraacetic acid on hematologic values in Eastern box turtles (Terrapene carolina carolina). J Zoo Wildl Med. 2021;51(4):999-1006. doi: 10.1638/2020-0109. PMID: 33480581.
13. Landt M, Hortin GL, Smith CH, et al. Interference in ionized calcium measurements by heparin salts. Clin Chem. 1994;40(4):565-570.
14. López-Olvera JR, Montané J, Marco I, et al. Effect of venipuncture site on hematologic and serum biochemical parameters in marginated tortoise (Testudo marginata). J Wildl Dis. 2003;39(4):830-836. doi: 10.7589/0090-3558-39.4.830. PMID: 14733278.
15. Martinez-Jiménez D, Hernandez-Divers SJ, Floyd TM, et al. Comparison of the effects of dipotassium ethylenediaminetetraacetic acid and lithium heparin on hematologic values in yellow-blotched map turtles (Graptemys flavimaculata). J Herp Med Surg. 2007;17(2):36–41. doi: 10.5818/1529-9651.17.2.36.
16. Mayer J, Knoll J, Innis C, Mitchell MA. Characterizing the hematologic and plasma chemistry profiles of captive Chinese water dragons, Physignathus cocincinus. J Herp Med Surg. 2005; 15(3):45–52. doi: https://doi.org/10.5818/1529-9651.15.3.45.
17. Mitchell MA. Reptile biochemistries. Proc International Conference on Avian, Herpetological and Exotic Mammal Medicine. 2013:22-23.
18. Mitchell MA. Managing the reptile patient in the veterinary hospital: Establishing a standard of care model for nontraditional species. J Exotic Pet Med. 2010;19(1):56-72. doi: 10.1053/j.jepm.2010.01.015.
19. Muro J, Cuenca R, Pastor J, et al. Effects of lithium heparin and tripotassium EDTA on hematologic values of Hermann’s tortoises (Testudo hermanni). J Zoo Wildl Med. 1998;29(1):40-44.
20. Myers DA, Mitchell MA, Fleming G, et al. Determining the value of bovine albumin as a blood cell stabilizer for pancake tortoise, Malacochersus tornieri, blood smears. J Herp Med Surg. 2008;18(3–4):95–99. doi: 10.5818/1529-9651.18.3-4.95.
21. Nevarez J. Spotlight on anesthesia and analgesia in reptiles. LafeberVet website. Available at https://lafeber.com/vet/spotlight-anesthesia-analgesia-reptiles/. Accessed April 23, 2022.
21b. Nevarez J. Lizards. In: Mitchell MA, Tully TN (eds). Manual of Exotic Pet Practice. Saunders Elsevier; St. Louis; 2009:179-180.
22. Pagés T, Peinado VI, Viscor G. Seasonal changes in hematology and blood chemistry of the freshwater turtle Mauremys caspica leprosa. Comp Biochem Physiol A. 1992;103(2):275–278.
23. Pendl H. Avian and reptilian haematology Proc International Conference on Avian, Herpetological and Exotic Mammal Medicine. 2015:177-178.
24. Perpiñán D, Armstrong DL, Dórea F. Effect of anticoagulant and venipuncture site on hematology and serum chemistries of the spiny softshell turtle (Apalone spinifera). J Herpetological Med Surg. 2010;20(2-3):74-78. doi: 10.5818/1529-9651-20.2.74.
25. Proença LM. Blood sampling and intravenous access in exotic species. Proc International Conference on Avian, Herpetological and Exotic Mammal Medicine. 2015:122-126.
26. Saggese MD. Clinical approach to the anemic reptile. J Exotic Pet Med 2009;18(2):98-111. doi: 10.1053/j.jepm.2009.04.003.
27. Tappin S, Rizzo F, Dodkin S, et al. Measurement of ionized calcium in canine blood samples collected in prefilled and self-filled heparinized syringes using the i-STAT point-of-care analyzer. Vet Clin Pathol. 2008;37(1):66-72. doi: 10.1111/j.1939-165X.2008.00001.x. PMID: 18366547.
28. van Berkel M, Scharnhorst V. Electrolyte-balanced heparin in blood gas syringes can introduce a significant bias in the measurement of positively charged electrolytes. Clin Chem Lab Med. 2011;49(2):249-52. doi: 10.1515/CCLM.2011.047. Epub 2010 Dec 14. PMID: 21143021.
Further reading
de la Navarre BJS. Current diagnostic techniques and therapeutic techniques in reptiles and amphibians. Proc International Conference on Avian, Herpetological and Exotic Mammal Medicine 2015:73-74
de la Navarre BJ. Common procedures in reptiles and amphibians. Vet Clin North Am Exot Anim Pract. 2006;9(2):237-267, vi. doi: 10.1016/j.cvex.2006.04.002. PMID: 16759946.
Heatley JJ. Box turtle (Terrapene spp.) hematology. J Exotic Pet Med 2010;19(2):160-164. doi: 10.1053/j.jepm.2010.06.002.
Glassman AR, Gamblin KM, Zachariah TT. Comparison of biochemistry values from plasma and lymph in Krefft’s river turtles (Emydura macquarii krefftii). J Herp Med Surg 2022;32(1):63-72. doi: 10.5818/JHMS-D-20-00017.
Lloyd M, Morris PJ. Phlebotomy techniques in crocodilians. Bulletin of the Association of Reptilian and Amphibian Veterinarians. 1999;9(3):12–14. doi: 10.5818/1076-3139.9.3.12.
Mader DR. Clinical pathology in reptiles: What do these results mean? Proc International Conference on Avian, Herpetological and Exotic Mammal Medicine. 2015:59-60.
Sykes JM 4th, Klaphake E. Reptile hematology. Vet Clin North Am Exot Anim Pract. 2015;18(1):63-82. doi: 10.1016/j.cvex.2014.09.011. PMID: 25421027.